
We, at the Nayak Research Group, an interdisciplinary Research Lab, believe in steady and active collaborations and are open to innovations while investigating novel concepts that underlie the complexities of our field. Our research is currently funded by both Government of India and Industries.
Dr. Sasmita Nayak (M.B.B.S., Ph.D.)
Associate Professor
Dr. Sasmita Nayak is a distinguished experimental biologist, primarily focused on developing sustainable antimicrobial materials aimed at preventing infections, with a specific emphasis on understanding the underlying mechanisms driving their biocidal properties. Such materials will have implications as antifouling, biocompatible and biocorrosion resistant materials in various in vitro and in vivo setups. In acknowledgment of her significant and enduring contributions to materials science and technology, Dr. Nayak was recently honored with the "Advanced Materials Innovation Award." This prestigious accolade represents the highest distinction conferred by the International Association of Advanced Materials, based in Stockholm, Sweden. Her research also encompasses elucidating the splicing regulatory mechanisms of the critical protein SufB in Mycobacterium tuberculosis and its applications in anti-TB drug development. She has successfully spearheaded multiple national and international research grants along with prominent industry research partners like NALCO Ltd., India and Tata Steel Ltd., India making substantial contributions to application-driven research. In 2023, she received the 'Women Leaders in Energy' award in recognition of her contributions to promoting cutting-edge material-based energy solutions as a co-founder of a pioneering renewable energy manufacturing company, KARMA Pvt. Ltd. Currently, Dr. Nayak holds the position of Associate Professor at the School of Biotechnology, KIIT-DU and serves as an Adjunct Associate Professor at Kalinga Institute of Medical Sciences, KIIT-DU, Bhubaneswar, India.



Research Overview
Multidiscriplinary research focus on antimicrobial functional materials and intein splicing towards drug therapy and environmental monitoring.
Nanomaterials as next generation antimicrobial composites/surfaces:
Nanomaterials such as silver nanoparticles and graphene-based composites are known to exhibit biocidal activities. However, interaction with the surrounding medium and supporting substrates can significantly influence this activity. Graphene, a carbon allotrope has unique structural and physicochemical properties, leading to the major focus of our research. Graphene and its derivates like Graphene oxide (GO) and reduced Graphene Oxide (rGO) are biocompatible in nature. Hence, their application in the biomedical sector is currently an intense area of research. Nayak group currently focuses on the antimicrobial properties and the mechanism of such antimicrobial activities of GO coatings on different metallic films. In one of the recent works, the observed activity is directly correlated to the electrical conductivity of the GO-metal systems; the higher the conductivity the better is the antibacterial activity. GO-metal substrate interactions serve as an efficient electron sink for the bacterial respiratory pathway, where electrons modify oxygen-containing functional groups on GO surfaces to generate reactive oxygen species (ROS). A concerted effect of non-oxidative electron transfer mechanism and consequent ROS mediated oxidative stress to the bacteria result in an enhanced antimicrobial action of naturally derived GO-metal films. This results can lead to new GO coated antimicrobial metal surfaces important for environmental and biomedical applications. A recently funded project involves examining the antimicrobial properties of different polymer coated substrates.














On the top, overlay images of E. coli cells exposed to different GO-Al substrates for a period of 2 hours. Green signal represents Live cells (Syto9) Red/yellow signal represents Dead cells (Propidium iodide). In the middle, FESEM images showing the degree of bacterial membrane damage. At the bottom, Detection of ROS production by staining with indicator dye DCFH-DA. Green signal represents generation of ROS following incubation of bacterial cells upon different GO-metal surfaces.



Reprinted from our research paper entitled "Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals" published in Advanced Materials (2018).
Mtu SufB Intein splicing towards Drug Therapy, Protein Purification, and Environmental Sensing:
The other research focus of our group is a fundamental understanding of splicing regulation of Mtu SufB intein. Inteins are intervening polypeptides that interrupt the functional domains of several important proteins across the three domains of life. Inteins excise themselves from the precursor protein, ligating concomitant extein residues in a process called protein splicing. Post-translational auto-removal of inteins remain critical for the generation of active proteins. The perspective of inteins in science is a robust field of research, however fundamental studies centralized upon splicing regulatory mechanisms are imperative for addressing more intricate issues. Mycobacterium tuberculosis (Mtu) carries an intein-bearing SufB protein, which is essential for Fe-S cluster biogenesis, especially during periods of stress, that ultimately determines virulence inside the host. Thus, SufB is a central and unique component of the mycobacterial SUF system and intein splicing is crucial for the functionality of SufB protein. We found that SufB protein is well conserved in mycobacteria, bacteria, and archaea during the evolutionary course. Intein insertion points may vary in some species, but the conservation of critical residues in SufB intein and extein regions do exist. Our current work focuses primarily on identifying the role(s) of conserved catalytic residues and environmental condition(s) on Mtu SufB intein splicing. This mechanism could provide a novel approach to develop anti-tubercular drugs through the regulation of splicing and the generation of an essential protein in pathogenic mycobacteria.

Schematic representation of different structural domains of the Mtu FL-SufB precursor. Important residues for the intein (numbered 1, 2, ...), N-extein (numbered −1, −2, ...) and C-extein (numbered +1, +2, ...) are shown at the top. The conserved N-extein histidines are H-5 (H248 in Mtu FL-SufB) and H-38 (H215 in Mtu Fl-SufB).
Reprinted from our research paper entitled "SufB intein splicing in Mycobacterium tuberculosis is influenced by two remote conserved N-extein histidines" published in Bioscience Reports (2022).

Schematic representation of intein splicing following a classical or canonical pathway. (i) Protein splicing involves 4 sequential nucleophilic displacements reactions catalyzed by Cys1, Cys1 and terminal Asn. 1. Formation of thioester generation by N-S acyl shift. 2. Generation of the branched intermediate by trans-esterification. 3. Resolution of the branched intermediate by Asn cyclization and intein excision. 4. Rearrangement of thioester to peptide bond give rise to ligated exteins(LE). ii) and iii) Off - pathway cleavage products. NC: N-terminal cleavage product; NE: N-extein; CC: C-terminal cleavage product; CE: C-Extein.
Reprinted from our research paper entitled "Metal effect on intein splicing: A review" published in Biochime (2021).


Structural model and proposed mechanism for N-cleavage reaction in Mtu FL-SufB precursor. The 3D model of Mtu FL-SufB precursor obtained in explicit solvent (left). Different domains and conserved catalytic residues are color coded as shown in the legend. Spatial arrangement of the N-terminal active site residues; Cys253 (C1) and Gly252 (G-1) along with His248 (H-5), His215 (H-38) and Asp217 (D-36) at the N-terminal cleavage site. On the right shows the proposed mechanism for N-terminal cleavage reaction at intein–extein junction.
Reprinted from our research paper entitled "SufB intein splicing in Mycobacterium tuberculosis is influenced by two remote conserved N-extein histidines" published in Bioscience Reports (2022).
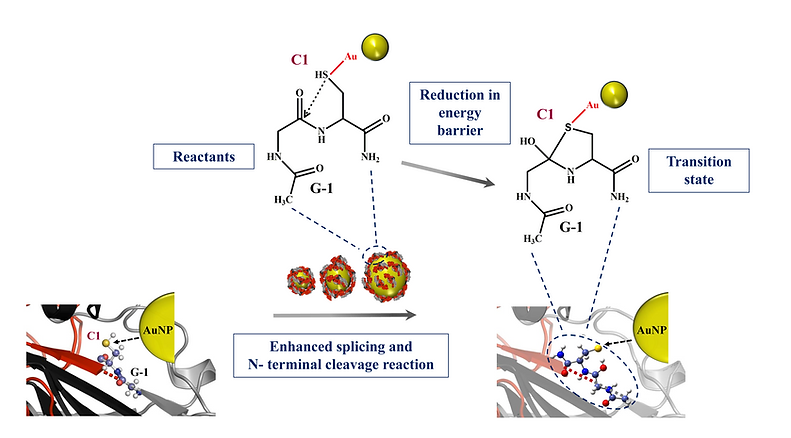
We present an efficient strategy for augmenting N-terminal cleavage and splicing reactions using AuNPs of varied sizes. Time-dependent refolding studies on Mtu SufB protein in the presence of 20 nm AuNPs showed an overall enhanced effect on N-terminal cleavage efficiency until 1 h and splicing efficiency after 4 h of the refolding reaction. Under similar experimental conditions, 10-nm AuNPs performed better than 5-nm AuNPs. Thus, we observe a size-dependent activity of AuNPs on Mtu SufB splicing and cleavage reactions. In agreement with the thiophilic nature of the gold nanoparticles, we have found a reduction in the energy barrier for the Gly-Cys system at the N-terminal splice junction that perhaps increased the N-terminal cleavage reaction and the overall splicing efficiency. Occurrence of heightened N-terminal cleavage reaction in Mtu SufB, as early as 10 min and conservation until 1 h under the influence of 20-nm gold nanoparticles can make it a valuable tool in the development of a novel protein purification strategy.
